Congratulations to Dr. Elijah Kuska on earning his Doctorate in Mechanical Engineering! Dr. Kuska’s PhD thesis dissertation was titled In Silico Techniques to Improve Understanding of Gait in Cerebral Palsy. Congratulations and best of luck as you move forward as an assistant teaching professor at the Colorado School of Mines!
Congratulations to Dr. Elijah Kuska on earning his Doctorate in Mechanical Engineering! Dr. Kuska’s PhD thesis dissertation was titled In Silico Techniques to Improve Understanding of Gait in Cerebral Palsy. Congratulations and best of luck as you move forward as an assistant teaching professor at the Colorado School of Mines!
MotorControl
AM Spomer, RZ Yan, MH Schwartz, KM Steele (2023) “Motor control complexity can be dynamically simplified during gait pattern exploration using motor control-based biofeedback”
Journal Article in Journal of Neurophysiology
Understanding how the central nervous system coordinates diverse motor outputs has been a topic of extensive investigation. Although it is generally accepted that a small set of synergies underlies many common activities, such as walking, whether synergies are equally robust across a broader array of gait patterns or can be flexibly modified remains unclear.
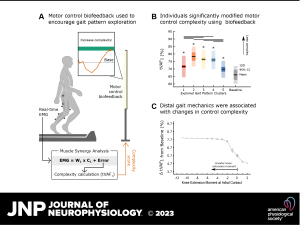 Aim: The aim of this study was to characterize the robustness of synergies to changing biomechanical constraints during walking. Specifically, we evaluated the extent to which nondisabled individuals could modulate both synergy structure and complexity while using motor control biofeedback to drive broad gait pattern exploration.
Aim: The aim of this study was to characterize the robustness of synergies to changing biomechanical constraints during walking. Specifically, we evaluated the extent to which nondisabled individuals could modulate both synergy structure and complexity while using motor control biofeedback to drive broad gait pattern exploration.
Methods: We evaluated the extent to which synergies changed as nondisabled adults (n = 14) explored gait patterns using custom biofeedback. Secondarily, we used Bayesian additive regression trees to identify factors that were associated with synergy modulation.
Results: Participants explored 41.1 ± 8.0 gait patterns using biofeedback, during which synergy recruitment changed depending on the type and magnitude of gait pattern modification. Specifically, a consistent set of synergies was recruited to accommodate small deviations from baseline, but additional synergies emerged for larger gait changes. Synergy complexity was similarly modulated; complexity decreased for 82.6% of the attempted gait patterns, but distal gait mechanics were strongly associated with these changes. In particular, greater ankle dorsiflexion moments and knee flexion through stance, as well as greater knee extension moments at initial contact, corresponded to a reduction in synergy complexity.
Interpretation: Taken together, these results suggest that the central nervous system preferentially adopts a low-dimensional, largely invariant control strategy but can modify that strategy to produce diverse gait patterns. Beyond improving understanding of how synergies are recruited during gait, study outcomes may also help identify parameters that can be targeted with interventions to alter synergies and improve motor control after neurological injury.
New & Noteworthy: We used a motor control-based biofeedback system and machine learning to characterize the extent to which nondisabled adults can modulate synergies during gait pattern exploration. Results revealed that a small library of synergies underlies an array of gait patterns but that recruitment from this library changes as a function of the imposed biomechanical constraints. Our findings enhance understanding of the neural control of gait and may inform biofeedback strategies to improve synergy recruitment after neurological injury.
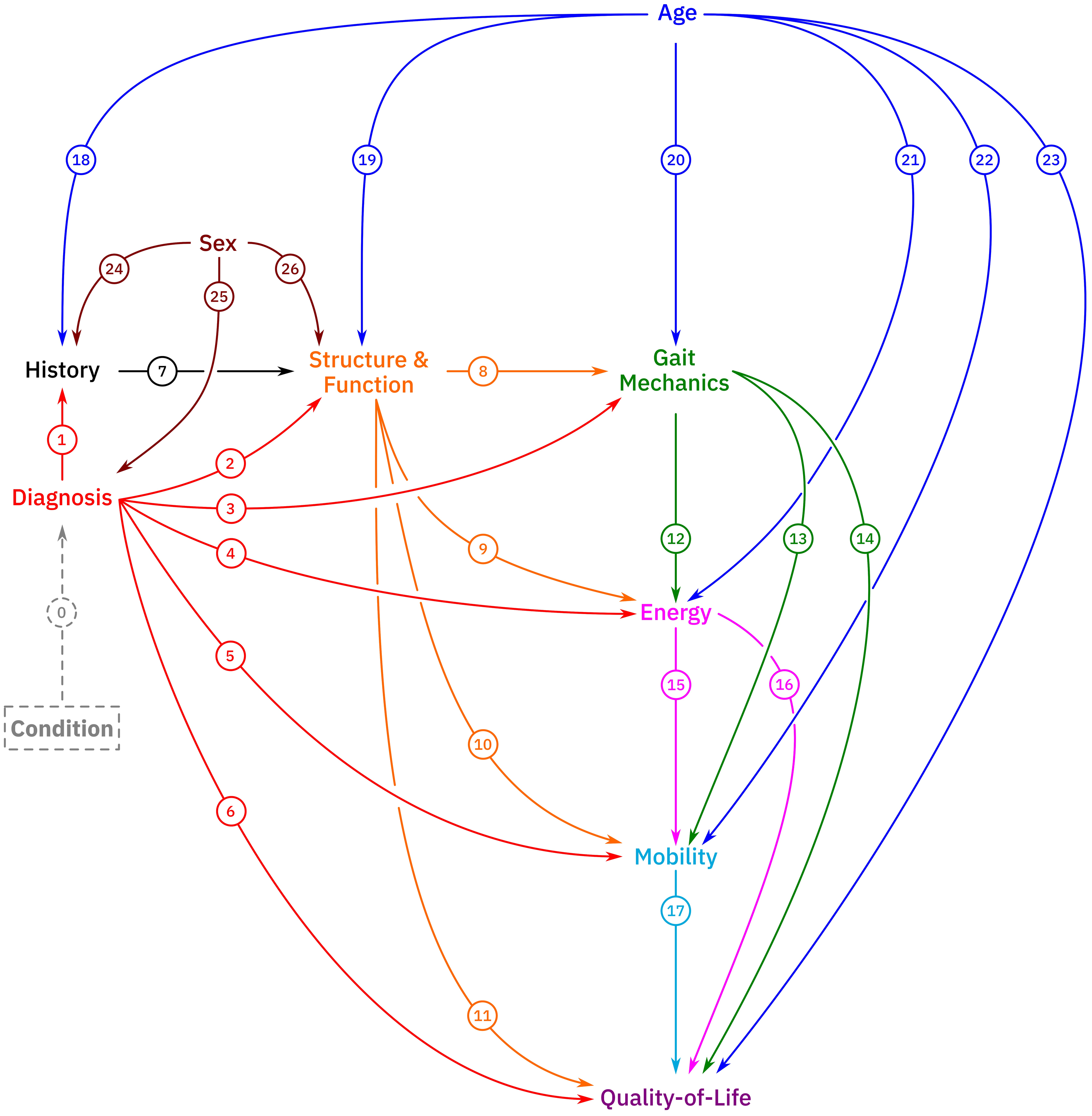
MH Schwartz, KM Steele, AJ Ries, AG Georgiadis, BA MacWilliams (2022) “A model for understanding the causes and consequences of walking impairments”
Journal Article in PLOS ONE:
Causal inference is inherently ambiguous since we cannot observe multiple realizations of the same person with different characteristics. Causal models must be evaluated through indirect means and reasoning.
Aim: The main objectives in conducting this study were to (1) propose a comprehensive model for quantifying the causes and consequences of walking impairments and (2) demonstrate the potential utility of the model for supporting clinical care and addressing basic scientific questions related to walking.
Method: This paper introduced a model consisting of 10 nodes and 23 primary causal paths and demonstrated the model’s utility using a large sample of gait data.
Results: The model was plausible, captured some well-known cause-effect relationships, provided new insights into others, and generated novel hypotheses requiring further testing through simulation or experiment.
Interpretation: This model is a proposal that is meant to be critically evaluated, validated or refuted, altered, and improved over time. Such improvements might include the introduction of new nodes, variables, and paths.
Introducing Dr. Alyssa Spomer!!
Congratulations to Dr. Alyssa Spomer on earning her Doctorate in Mechanical Engineering! Dr. Spomer’s PhD thesis dissertation was titled Evaluating multimodal biofeedback to target and improve motor control in cerebral palsy. Congratulations and best of luck as you move forward as a Clinical Scientist at Gillette Children’s. Best of luck in Minnesota!