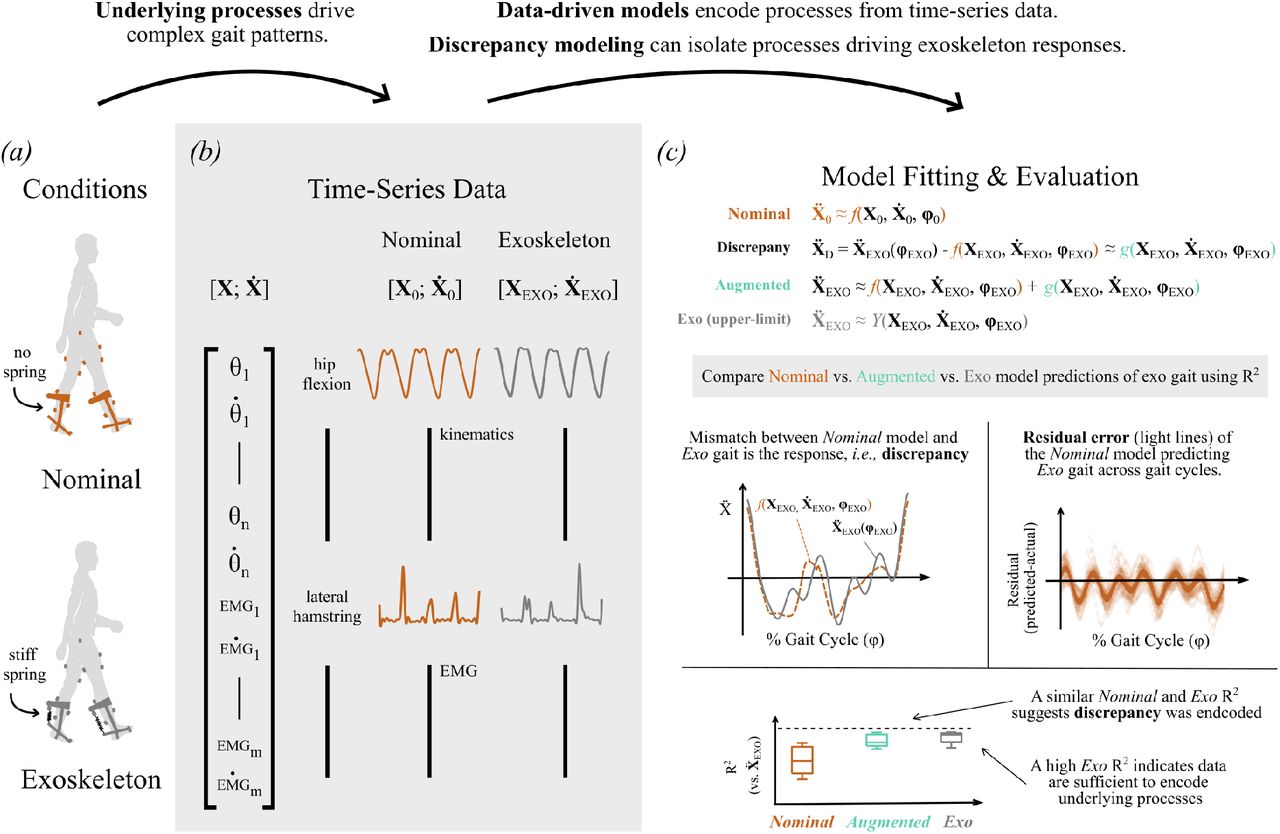
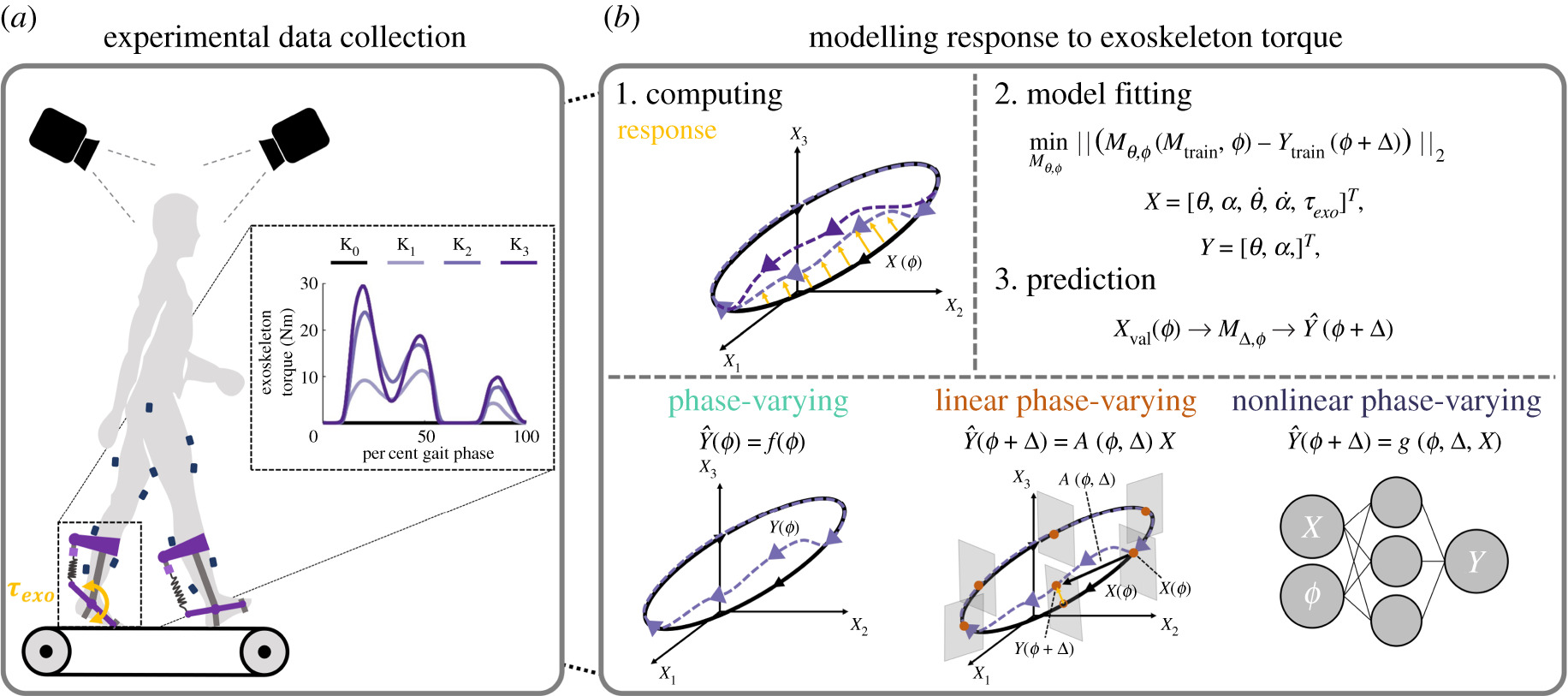
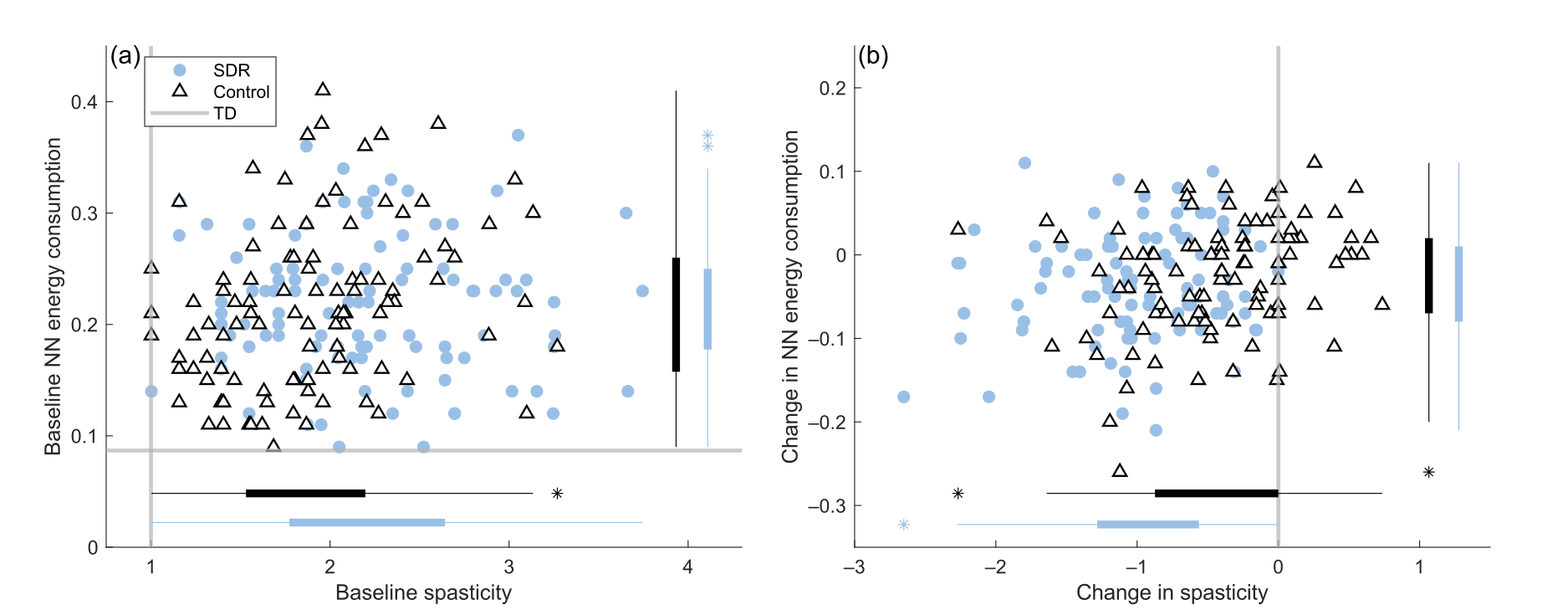
Journal Article in Frontiers in Human Neuroscience
Altered motor control is common in cerebral palsy (CP). Understanding how altered motor control affects movement and treatment outcomes is important but challenging due to complex interactions with other neuromuscular impairments. While regression can be used to examine associations between impairments and movement, causal modeling provides a mathematical framework to specify assumed causal relationships, identify covariates that may introduce bias, and test model plausibility.
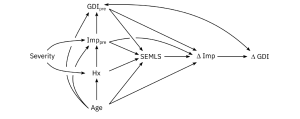 Aim: The goal of this research was to quantify the causal effects of altered motor control and other impairments on gait, before and after single-event multi-level orthopedic surgery (SEMLS).
Aim: The goal of this research was to quantify the causal effects of altered motor control and other impairments on gait, before and after single-event multi-level orthopedic surgery (SEMLS).
Methods: We evaluated the impact of SEMLS on change in Gait Deviation Index (ΔGDI) between gait analyses. We constructed our causal model with a Directed Acyclic Graph that included the assumed causal relationships between SEMLS, ΔGDI, baseline GDI (GDIpre), baseline neurologic and orthopedic impairments (Imppre), age, and surgical history. We identified the adjustment set to evaluate the causal effect of SEMLS on ΔGDI and the impact of Imppre on ΔGDI and GDIpre. We used Bayesian Additive Regression Trees (BART) and accumulated local effects to assess relative effects.
Results: We prospectively recruited a cohort of children with bilateral CP undergoing SEMLS (N = 55, 35 males, age: 10.5 ± 3.1 years) and identified a control cohort with bilateral CP who did not undergo SEMLS (N = 55, 30 males, age: 10.0 ± 3.4 years). There was a small positive causal effect of SEMLS on ΔGDI (1.70 GDI points). Altered motor control (i.e., dynamic and static motor control) and strength had strong effects on GDIpre, but minimal effects on ΔGDI. Spasticity and orthopedic impairments had minimal effects on GDIpre or ΔGDI.
Interpretation: Altered motor control did have a strong effect on GDIpre, indicating that these impairments do have a causal effect on a child’s gait pattern, but minimal effect on expected changes in GDI after SEMLS. Heterogeneity in outcomes suggests there are other factors contributing to changes in gait. Identifying these factors and employing causal methods to examine the complex relationships between impairments and movement will be required to advance our understanding and care of children with CP.
 This summer, the Steele Lab hosted undergraduate researcher, Amina El-Zatmah, from Santa Monica College. She finished up her 10-week summer Research Experience for Undergraduate (REU) by presenting at the 2023 Summer Undergraduate Research Symposium with the Center for Neurotechnology (CNT).
This summer, the Steele Lab hosted undergraduate researcher, Amina El-Zatmah, from Santa Monica College. She finished up her 10-week summer Research Experience for Undergraduate (REU) by presenting at the 2023 Summer Undergraduate Research Symposium with the Center for Neurotechnology (CNT).