Journal Article in Journal of NeuroEngineering & Rehabilitation
Background
Accelerometers have become common for evaluating the efficacy of rehabilitation for patients with neurologic disorders. For example, metrics like use ratio (UR) and magnitude ratio (MR) have been shown to differentiate movement patterns of children with cerebral palsy (CP) compared to typically-developing (TD) peers. However, these metrics are calculated from “activity counts” – a measure based on proprietary algorithms that approximate movement duration and intensity from raw accelerometer data. Algorithms used to calculate activity counts vary between devices, limiting comparisons of clinical and research results. The goal of this research was to develop complementary metrics based on raw accelerometer data to analyze arm movement after neurologic injury.
Method
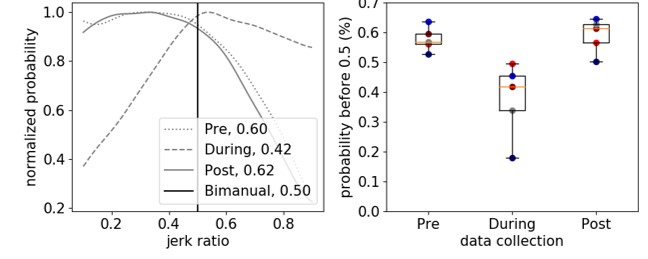
We calculated jerk, the derivative of acceleration, to evaluate arm movement from accelerometer data. To complement current measures, we calculated jerk ratio (JR) as the relative jerk magnitude of the dominant (non-paretic) and non-dominant (paretic) arms. We evaluated the JR distribution between arms and calculated the 50th percentile of the JR distribution (JR50). To evaluate these metrics, we analyzed bimanual accelerometry data for five children with hemiplegic CP who underwent Constraint-Induced Movement Therapy (CIMT) and five typically developing (TD) children. We compared JR between the CP and TD cohorts, and to activity count metrics.
Results
The JR50 differentiated between the CP and TD cohorts (CP = 0.578±0.041 before CIMT, TD = 0.506±0.026), demonstrating increased reliance on the non-dominant arm for the CP cohort. Jerk metrics also quantified changes in arm use during and after therapy (e.g., JR50 = 0.378±0.125 during CIMT, 0.591 ± 0.057 after CIMT). The JR was strongly correlated with UR and MR (r = -0.92, 0.89) for the CP cohort. For the TD cohort, JR50 was repeatable across three data collection periods with an average similarity of 0.945±0.015.
Conclusions
Acceleration-derived jerk captured differences in motion between TD and CP cohorts and correlated with activity count metrics. The code for calculating and plotting JR is open-source and available for others to use and build upon. By identifying device-independent metrics that can quantify arm movement in daily life, we hope to facilitate collaboration for rehabilitation research using wearable technologies.
Code
The algorithm for calculating jerk ratio, as well as user-friendly code to produce plots similar to the figure above are provided open-source as Python 3.6 code as a Python Jupyter Notebook within Google Colab. With this resource, research groups can use existing or newly created data from accelerometers to analyze jerk ratio as a complementary metric to existing measures, enabling comparison between research studies or centers that may rely on different sensors and activity count algorithms.