Journal Article in Journal of Biomechanics
Cerebral palsy (CP) is a neurologic injury that impacts control of movement. Individuals with CP also often develop secondary impairments like weakness and contracture. Both altered motor control and secondary impairments influence how an individual walks after neurologic injury. However, understanding the complex interactions between and relative effects of these impairments makes analyzing and improving walking capacity in CP challenging.
 Aim: The purpose of this study was to investigate the interactions between neuromuscular impairments and gait in CP.
Aim: The purpose of this study was to investigate the interactions between neuromuscular impairments and gait in CP.
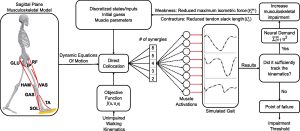
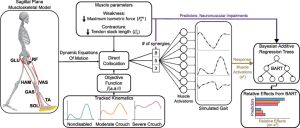
Methods: We used a sagittal-plane musculoskeletal model and neuromuscular control framework to simulate crouch and nondisabled gait. We perturbed each simulation by varying the number of synergies controlling each leg (altered control), and imposed weakness and contracture. A Bayesian Additive Regression Trees (BART) model was also used to parse the relative effects of each impairment on the muscle activations required for each gait pattern.
Results: By using these simulations to evaluate gait-pattern specific effects of neuromuscular impairments, we identified some advantages of crouch gait. For example, crouch tolerated 13 % and 22 % more plantarflexor weakness than nondisabled gait without and with altered control, respectively. Furthermore, BART demonstrated that plantarflexor weakness had twice the effect on total muscle activity required during nondisabled gait than crouch gait. However, crouch gait was also disadvantageous in the presence of vasti weakness: crouch gait increased the effects of vasti weakness on gait without and with altered control.
Interpretation: These simulations highlight gait-pattern specific effects and interactions between neuromuscular impairments. Utilizing computational techniques to understand these effects can elicit advantages of gait deviations, providing insight into why individuals may select their gait pattern and possible interventions to improve energetics.